In the rapidly evolving landscape of healthcare and pharmaceuticals, the role of Contract Research Organizations (CROs) has become increasingly pivotal. In India, the emergence of CROs has played a significant role in transforming the clinical research ecosystem, providing essential services to biopharmaceutical companies, medical device manufacturers, and other stakeholders in the healthcare sector. This article delves into the multifaceted role of CRO in India – Cliniexperts, highlighting their contributions to the clinical research field, regulatory environment, and the overall advancement of healthcare in the country.
Understanding CROs
Contract Research Organizations (CROs) are companies that provide outsourced research services to pharmaceutical, biotechnology, and medical device industries. These services can range from preclinical research and clinical trial management to data management and regulatory affairs. By leveraging specialized expertise, CROs enable their clients to expedite the development process of new therapies, improve cost efficiency, and reduce the overall time to market.
The Growth of CROs in India
The Indian clinical research market has witnessed exponential growth over the past decade. With a rich pool of diverse patient populations, skilled professionals, and cost-effective operations, India has emerged as a preferred destination for conducting clinical trials. The increasing complexity of drug development, coupled with the rising demand for innovative therapies, has fueled the growth of CROs in the region. This expansion has been supported by a favorable regulatory environment, which has evolved to facilitate the efficient conduct of clinical trials.
Key Functions of CROs in India
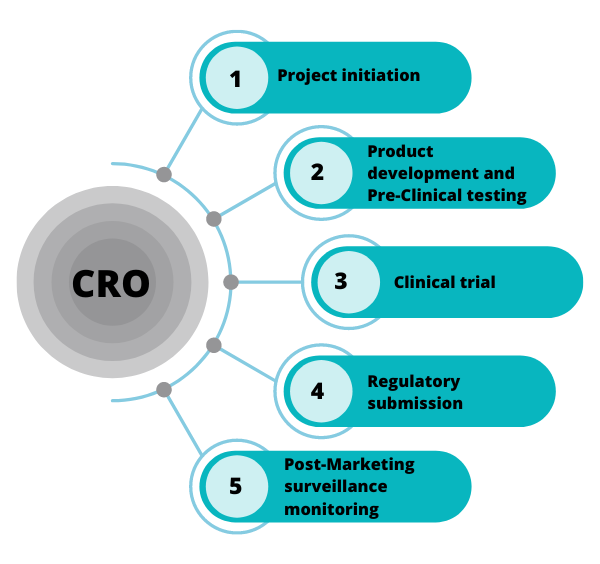
CROs in India play several crucial roles, including:
- Clinical Trial Management: CROs are responsible for planning, executing, and managing clinical trials on behalf of their clients. This includes site selection, patient recruitment, monitoring trial progress, and ensuring compliance with regulatory guidelines. Their expertise allows sponsors to focus on their core competencies while outsourcing the complex logistics of trial management.
- Regulatory Affairs: Navigating the regulatory landscape is a significant challenge for many biopharmaceutical companies. CROs provide critical support in ensuring compliance with the regulations set forth by the Central Drugs Standard Control Organization (CDSCO) and other relevant authorities. They assist clients in preparing and submitting regulatory documentation, facilitating approvals, and maintaining adherence to Good Clinical Practices (GCP).
- Data Management and Analysis: Accurate and timely data collection is vital for the success of clinical trials. CROs employ sophisticated data management systems to capture, analyze, and report clinical data. They ensure data integrity and facilitate the statistical analysis required for regulatory submissions and publication in scientific journals.
- Patient Recruitment and Retention: One of the biggest challenges in clinical trials is recruiting and retaining participants. CROs leverage their extensive networks and local knowledge to implement effective patient recruitment strategies. They often utilize digital marketing, community outreach, and collaborations with healthcare professionals to enhance participant engagement and retention.
- Post-Marketing Surveillance: After a product is launched, CROs assist in monitoring its performance in real-world settings. They conduct post-marketing studies to assess the long-term safety and efficacy of therapies, ensuring that any adverse effects are identified and reported promptly.
Impact of CROs on the Indian Healthcare Ecosystem
The presence of CROs in India has far-reaching implications for the healthcare landscape:
- Accelerating Drug Development: By streamlining clinical trials and enhancing efficiency, CROs help bring innovative drugs and therapies to market faster. This acceleration is crucial for addressing unmet medical needs and improving patient outcomes.
- Economic Growth: The growth of CROs contributes to job creation and economic development in India. With increasing demand for clinical research services, the sector is attracting skilled professionals, further boosting the economy.
- International Collaboration: Indian CROs are increasingly collaborating with international pharmaceutical companies, positioning India as a key player in the global clinical research arena. This collaboration fosters knowledge exchange and promotes the adoption of best practices in clinical research.
- Enhancing Research Quality: CROs are committed to maintaining high standards of quality in clinical research. By adhering to international guidelines and conducting rigorous monitoring, they contribute to the credibility of clinical trial data generated in India.
Challenges Faced by CROs in India
Despite their significant contributions, CROs in India face several challenges, including:
- Regulatory Hurdles: While the regulatory environment has improved, navigating the complexities of approvals can still be time-consuming and cumbersome. CROs must stay abreast of evolving regulations and ensure compliance to avoid delays.
- Competition: The growing number of CROs in India has led to intense competition. To differentiate themselves, CROs must continually innovate and enhance their service offerings.
- Talent Shortage: As the clinical research industry expands, there is an increasing demand for skilled professionals. The shortage of trained personnel can hinder the growth of CROs and impact the quality of research.
Conclusion
The role of Contract Research Organizations in India is integral to the advancement of clinical research and the overall healthcare ecosystem. By providing essential services that streamline the drug development process, CROs not only enhance efficiency but also contribute to economic growth and improved patient outcomes. As the demand for innovative therapies continues to rise, the importance of CROs in India will only increase, making them a vital component of the healthcare landscape.
For further information about how CROs are shaping the future of clinical research in India, explore the services offered by CRO in India – Cliniexperts. Their commitment to excellence and innovation in clinical research positions them at the forefront of the industry, ensuring that the potential of Indian healthcare is fully realized.
